- Home
- Vesicular Arbuscular Mycorrhiza
Transform Your Garden into a Personal Paradise!
Beyond the Root: How Mycorrhizal Fungi Lead a Microbial Network to Healthier Soil
Mycorrhizal fungi are the cornerstone of the rhizosphere's "Wood Wide Web," coordinating with bacteria and protists in a powerful nutrient exchange. Learn how this partnership is key to plant health, soil carbon storage, and sustainable farming.
Beneath every thriving plant lies a hidden economy—one where currencies of carbon, nitrogen, and nutrients flow through a living network so sophisticated it makes our digital world seem simple.
We often hear about mycorrhizal fungi, nature's famous root partners, but they are just one actor in a much larger ensemble.
Welcome to the rhizosphere: the bustling, millimeter-thin zone around plant roots where fungi, bacteria, protists, and countless microbes don't just coexist—they collaborate with a precision that sustains entire ecosystems, builds climate resilience, and even powers a new kind of economy: the soil carbon market.
The Rhizosphere: Not Just Dirt, but a Living Marketplace
Picture the soil around a root not as dirt, but as a vibrant, microscopic city. The root is the central plaza, exuding root exudates—a rich cocktail of sugars, acids, and compounds that act like an open invitation and currency. This is where the collaboration begins.
The Key Players and Their Roles:
- Plant Roots: The "Bankers" and architects. They invest up to 30% of their photosynthesized carbon into the soil to hire their microbial workforce.
- Mycorrhizal Fungi: The "Distribution Network." Their vast mycelial highways transport water and nutrients (like phosphorus) to plants in exchange for carbon. They connect entire plant communities in a "Wood Wide Web."
- Bacteria: The "Specialized Processors." They fix atmospheric nitrogen, solubilize minerals, decompose organic matter, and produce growth-promoting hormones and natural antibiotics. Some, like rhizobia, form direct partnerships with legumes.
- Protists: The "Regulators and Recyclers." These microscopic predators graze on bacteria, releasing locked-up nitrogen and other nutrients in a process called the "microbial loop." They keep the bacterial community productive and diverse.
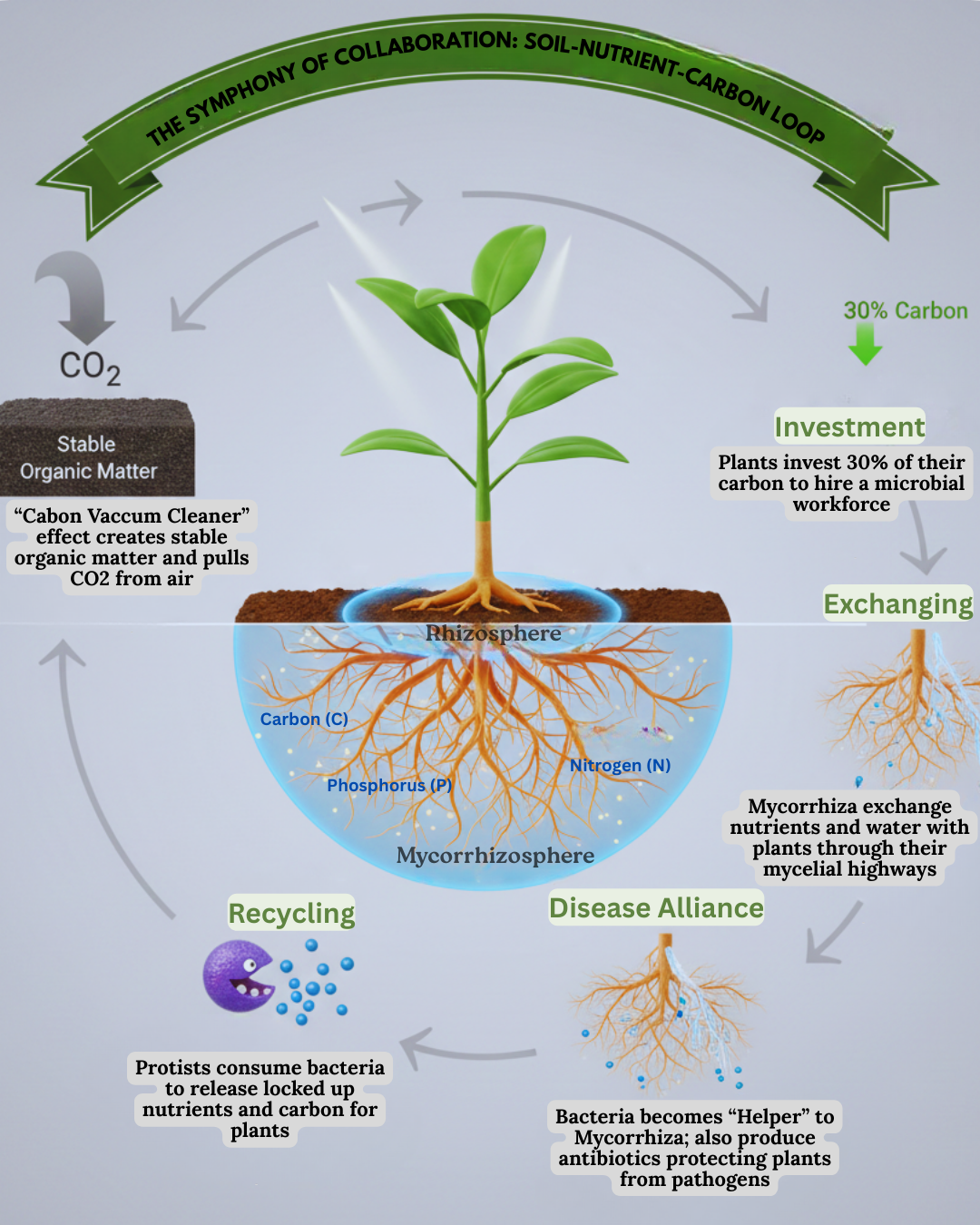
The Symphony of Collaboration: How the Web Functions?
This isn't a collection of independent organisms; it's a tightly integrated system where each member's survival boosts the others.
1. The Nutrient Exchange Cycle:
The plant pays fungi and bacteria with carbon (sugars). Bacteria process raw materials. Protists then consume bacteria, releasing plant-ready nutrients right onto the fungal highways. Fungi deliver these nutrients back to the plant. The plant, now healthier, photosynthesizes more, funding the next cycle.
2. The Defense Alliance:
A diverse rhizosphere microbiome is a plant's best immune system. Bacteria produce antibiotics. Mycorrhizal fungi form a physical barrier on roots. Predatory protists and nematodes consume pathogenic fungi and bacteria. Together, they "crowd out" disease.
3. The Soil Structure Brigade:
Fungal hyphae weave through soil particles, binding them into stable aggregates. Bacteria produce sticky glues (polysaccharides). This creates pore spaces for air, water, and root growth—transforming compacted dirt into resilient, spongy soil.
How This Tiny World Helps Our Big World (Including Climate!) ?
This teamwork doesn’t just help plants—it can pull carbon out of the air and lock it safely in the soil. Here’s how:
- Plants take CO₂ from the air.
- They send carbon down to their roots to feed microbes.
- Microbes use some carbon, but a lot of it gets stored in the soil as stable organic matter (thanks to fungal networks and microbial “leftovers”).
- This process makes soil richer and acts like a carbon vacuum cleaner for the atmosphere.
From Collaboration to Credits:
Regenerative agricultural practices (no-till, cover cropping, diverse rotations, compost) explicitly aim to nurture this rhizosphere web. By doing so, farmers can:
- Increase the amount of carbon sequestered in their soil.
- Have this sequestration measured and verified by third parties.
- Earn soil carbon credits sold to companies or governments to offset their emissions.
This creates a powerful financial incentive to farm in a way that supports the very microbial collaborations that underpin ecosystem health. It’s a market-driven revolution rooted in biology.
How to Nurture the Web: A Guide for Gardeners, Farmers?
You can’t create this web by force, but you can create the conditions for it to flourish.
Do`s:
- Feed the Network: Add diverse organic matter (compost, mulch, cover crop roots). This is the universal fuel.
- Diversify: Plant polycultures. Different root exudates support different microbes, strengthening the entire web.
- Minimize Disturbance: Reduce or eliminate tillage. It destroys fungal networks and soil aggregates.
- Keep it Covered: Bare soil is a dead zone. Living roots and mulch maintain the habitat.
Avoid:
- Broad-Spectrum Chemicals: Pesticides and fungicides are often "weapons of mass destruction" for this delicate community.
- Synthetic Nitrogen Overload: It can shut down natural nitrogen-fixing partnerships and acidify the soil.
- Compaction: It destroys the pore spaces that are the microbial habitat.
The Big Picture
Mycorrhizal fungi are great, but the real power is in the teamwork—the whole web of life in the rhizosphere. When we support this teamwork, we get:
- Healthier plants with less work
- Better soil that holds water
- Fewer pests and diseases
- More nutritious food
- A real, natural solution to climate change
Good gardening—and good farming—is less about controlling nature, and more about supporting the partnerships that already exist. When we feed the life in the soil, the soil feeds us back, in more ways than one.
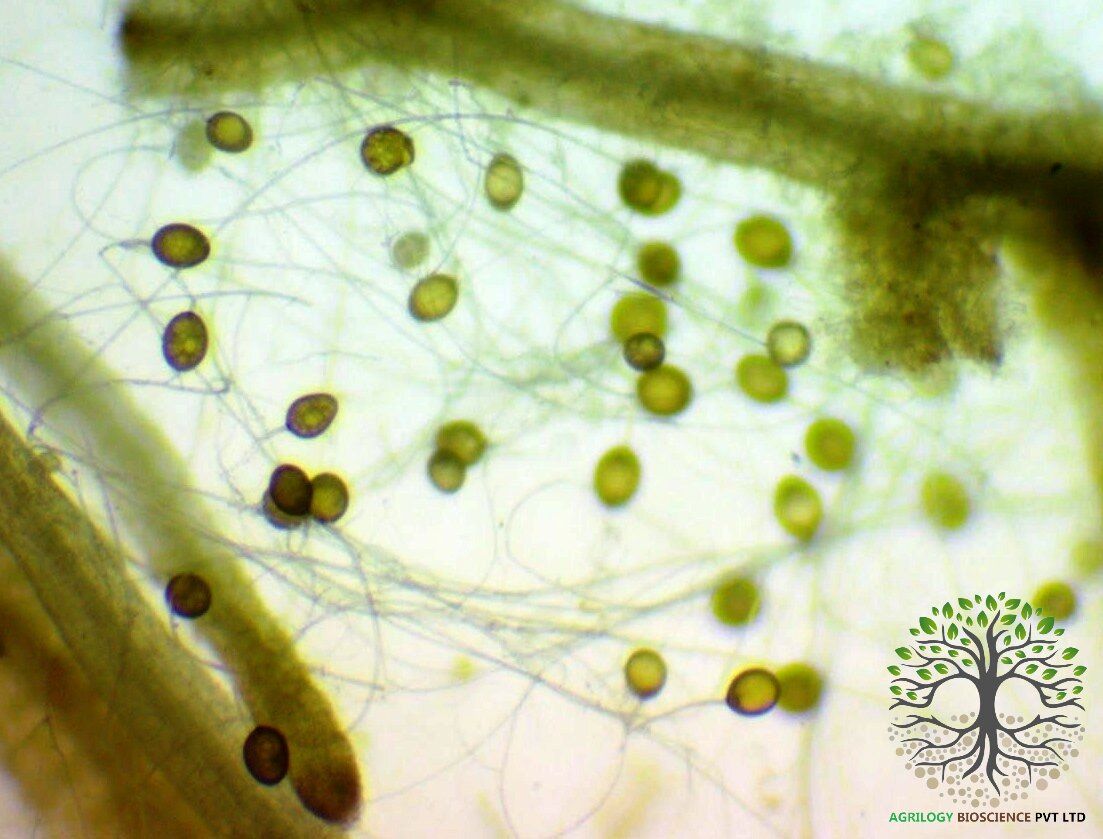
The Clustered Code: Deciphering the Secret Life of Mycorrhizal Spores
If you’ve ever peeked through a microscope at arbuscular mycorrhizal (AM) fungi spores, you’ve likely seen them: not just solitary spheres, but intriguing clusters where spores appear glued together like microscopic bunches of grapes. For years, these aggregated spores—particularly of the ubiquitous species Glomus intraradices (now classified within the Rhizophagus genus)—were often counted as one unit or overlooked. But emerging science reveals that this "clustering" is not a random accident. It's a sophisticated survival strategy with profound ecological implications. Let's dive into the sticky, clever world of clustered endomycorrhizal spores.
The Microscopic Glue: How Spores Stick Together?
First, the mechanics. How are these spores so firmly aggregated?
The binding agent is a complex matrix of extraradical mycelial networks and glycoprotein-based secretions. Think of it not as a simple adhesive, but as a living, “biological hydrogel”.
- The Hyphal "Backbone": Often, spores are formed at the tips or along the length of the extraradical hyphae (the fungal network outside the plant root). Instead of detaching, the hyphae connecting them thicken, melanize, and persist, forming a durable structural scaffold that physically links the spores.
- The Secreted "Bio-Glue": As spores mature, they and their supporting hyphae exude a cocktail of glycoproteins, mucilaginous polysaccharides, and hydrophobins. This secretion hardens upon dehydration, forming a tough, protective crust that cements the entire assembly—spores, hyphae, and soil particles—into a single, cohesive unit. This crust is key to the cluster's resilience.
Why Cluster? The Ecological & Physiological Genius of Glomus intraradices
For Glomus intraradices, a champion of crop and grassland systems, spore clustering is a masterclass in evolutionary adaptation.
1. The Ecological - Propagule Bank Microsite:
- Desiccation Defence: The shared, hardened glycoprotein matrix significantly reduces the surface area exposed to the environment, minimizing water loss. It creates a buffered microclimate around the spores, protecting them from drought.
- Predator & Pathogen Deterrence: The same tough, often melanised crust that prevents water loss also acts as a physical barrier against grazing by soil micro arthropods and invasion by parasitic fungi. It's the spore's version of a castle wall.
- Nutrient Reservoir & Hub: A cluster isn't just spores; it's a package of stored carbon, lipids, and nutrients. This shared "communal pantry" may allow for resource redistribution, potentially aiding weaker spores within the unit. The interconnected hyphae can also function as a pre-established network, giving new germination an instant head start.
2. The Physiological Powerhouse:
- Synergistic Germination Signals: There is compelling evidence that spores in a cluster can engage in cross-talk. A spore initiating germination may release chemical signals (e.g., strigolactones in response to root exudates) that can stimulate or prime neighbouring spores in the cluster, leading to coordinated, potentially more effective colonization events.
- Adaptive Diversification (of Dormancy): Not all spores in a cluster germinate simultaneously. This asynchronous germination is a critical risk-management strategy. If one spore germinates into unfavorable conditions and fails, others remain dormant, preserving the genetic lineage. It ensures the fungus doesn't put all its eggs in one basket.
The Germination Paradox: Does Clustering Help or Hinder?
This is the central question. The answer is nuanced: clustering delays but optimizes germination.
- The Delay: The physical barrier of the crust and the need to re-hydrate a larger, denser structure mean that clustered spores often germinate more slowly than solitary spores. They require a stronger, more persistent stimulus (like continuous root exudates) to initiate the process.
- The Ultimate Advantage: This delay is strategic. It acts as a dormancy filter, ensuring germination only occurs when conditions are truly favourable and a host root is reliably nearby. When they do germinate, they benefit from the "synergistic signals" and pre-built hyphal connections, potentially leading to more robust and successful colonization. The cluster ensures quality over speed.
A Call to Action: Why Specialists MUST Reconsider Spore Counting
This brings us to a critical methodological point. For mycorrhizal ecologists, agronomists, and inoculum producers, treating a spore cluster as "one" is a significant error. Here’s why:
- Inaccurate Propagule Potential: A cluster of 10 spores has 10x the genetic potential and nutrient reserves of a single spore. Counting it as one propagule drastically underestimates the true infectious potential of the soil or inoculum.
- Skewed Biodiversity & Abundance Data: In ecological surveys, failing to dis-aggregate clusters lead to severe underestimation of spore density and can distort comparisons between species (some cluster more than others) or between management practices.
- Misjudging Inoculum Quality: The proportion of clustered vs. solitary spores in a commercial inoculum could be a key quality indicator. A high cluster ratio suggests a product designed for resilience and long-term survival, while a high solitary spore count might indicate faster, but less persistent, colonization.
Best Practice: The specialist's approach should involve gentle crushing of samples in a weak surfactant solution to break up clusters before counting, providing a true measure of total spore numbers. Documenting the degree of clustering separately adds a valuable layer of ecological insight.
“Taken together, the physical structure, biochemical signals, and germination patterns indicate that the spore cluster functions as a unified survival module.".
Clustering represent a shift in how we view these fungi—not as collections of individual spores, but as complex, cooperative societies engineered for persistence.
Their sticky glue is the mortar of a survival fortress, their delayed germination a mark of wisdom, and their continued underestimation in counts a gap in our understanding.
By acknowledging and studying these clustered formations in their full complexity, we unlock a deeper appreciation of mycorrhizal ecology and move closer to harnessing their full power for sustainable agriculture and ecosystem restoration.
“Look closer. Count smarter. The future of soil health depends on such microscopic details.”
How to Make Biologicals Work? Optimizing the Soil-Root-Microbe System!
The promise of agricultural biologicals—from nitrogen-fixing bacteria to mycorrhizae fungi—is transforming modern farming. However, their success is not guaranteed by application alone. Unlike chemical inputs, these living products are sensitive performers in the complex realm of the soil and rhizosphere. Their efficacy depends on a symphony of environmental factors working in harmony. Here, we break down the core scientific parameters and management strategies that determine whether your biological investment will flourish or falter.
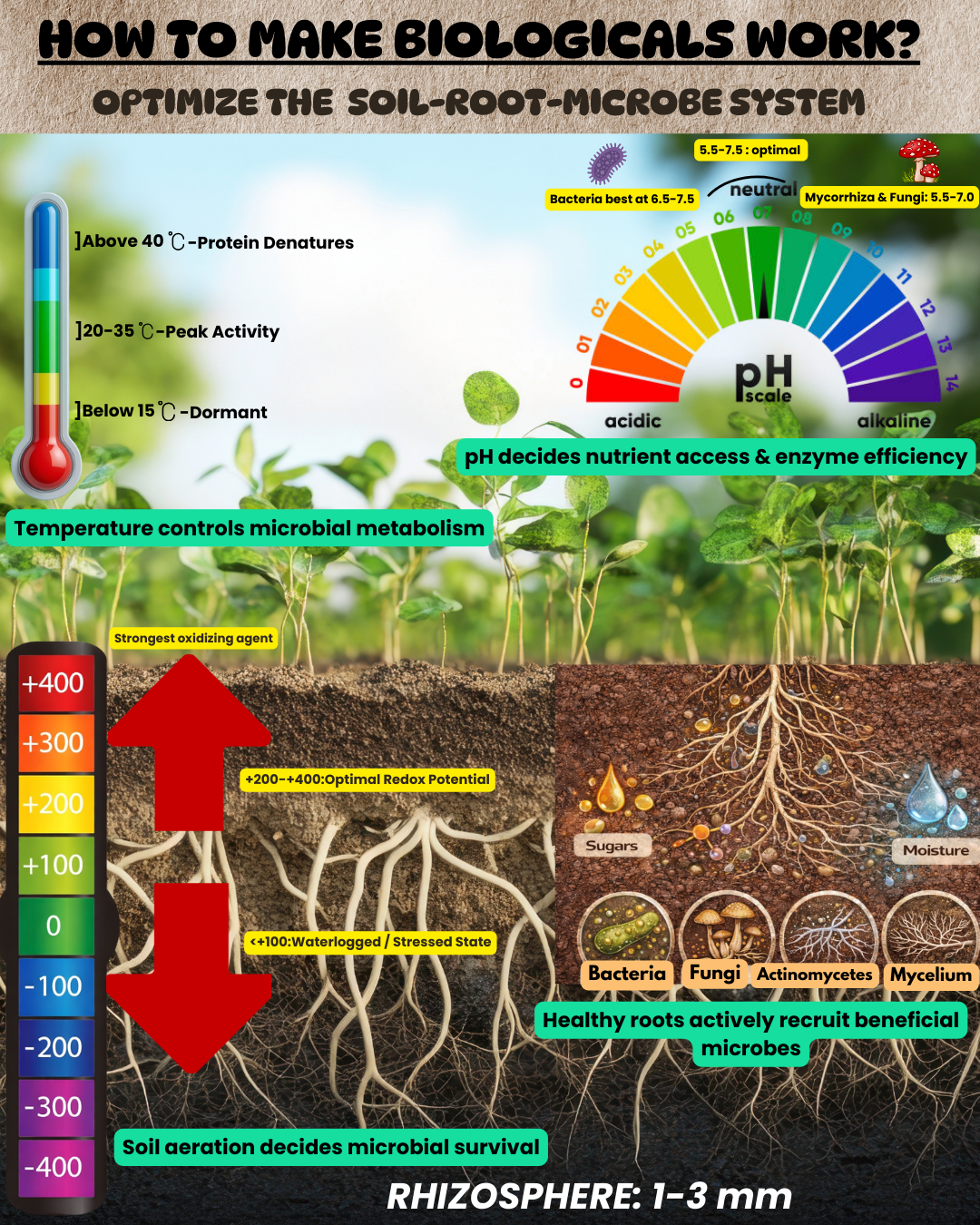
The Foundational Trio: pH, Redox Potential and Temperature
Think of these as the non-negotiable core abiotic driversfor your microbial workforce.
A. Redox Potential (Eh): The Breath of the Soil
Redox potential measures soil aeration, essentially telling you if your soil is gasping for air or breathing easily.
- Physiological Optimum: +200 to +400 mV for most beneficial aerobes.
- Microbial Preferences: Nitrogen-fixing Rhizobium needs well-aerated soil (Eh > +300 mV) to form nodules. PSB Pseudomonas operates well at moderate levels (+100 to +300 mV). Notably, while some anaerobes function at negative Eh, they are less common in standard biologicals.
- Key Insight: A waterlogged, low-Eh (< +100 mV) environment will suffocate many aerobic Plant Growth-Promoting Rhizobacteria (PGPR), though it may be less detrimental to certain fungi.
B. pH: The Acidity Balance
pH dictates nutrient availability and microbial membrane stability.
- Bacterial Preference: Thrive in neutral to slightly alkaline soils (pH 6.5-7.5), with exceptions like acid-tolerant strains.
- Fungal Preference: Enjoy a broader, slightly more acidic range (pH 5.5-7.5), with mycorrhizae performing optimally at pH 5.5-7.0.
- Critical Impact: pH directly influences enzyme activity and the efficiency of siderophores—the iron-scavenging molecules produced by many biocontrol agents.
C. Temperature: The Metabolic Thermostat
Temperature controls microbial activity and protein integrity.
- Optimal Range: 20-35°C (mesophilic range) for most products.
- Critical Thresholds: Activity significantly slows below 15°C, while sustained heat above 40°C can denature proteins in many PGPRs.
- Strategy: Match the inoculant to the season. Seek out psychrotolerant strains for early spring or fall applications and thermotolerant strains for summer use.
The Plant's Role: Root Architecture is Everything
The plant is not a passive recipient but an active regulator of its rhizosphere microbiome through its root architechture.
- Root Surface Area: Finer root systems create more sites for colonization.
- Root Exudates: This is the plant's chemical communication. Legumes secrete flavonoids to attract Rhizobium, while cereals release malic acid to beckon Bacillus subtilis. The quantity and quality of these exudates drive microbial chemotaxis.
- Root Hair Density: This is often the frontline for bacterial colonization—higher density means more entry points.
- Root Depth: Shallow root systems favour mycorrhiza partnerships, while deeper roots may require strategically placed inoculants.
Building a Favourable Soil Ecosystem
Beyond the core trio, a thriving soil ecosystem sets the stage for success.
A. Physical & Chemical Properties:
- Aim for loamy soils with good porosity (40-60% pore space) to allow microbial movement.
- Maintain organic matter above 2% to provide carbon and buffer changes.
- A C:N ratio of 20:1 to 30:1 is optimal. Avoid excessive nitrogen or phosphorus, which can inhibit biological N-fixation and P-solubilization.
- Ensure low salinity (EC < 2 dS/m) and a Cation Exchange Capacity > 10 cmol⁺/kg for nutrient retention.
B. The Rhizosphere Hotspot:
This 1-3 mm zone around the root is the action centre. Manage for:
- Exudate Profiles: A mix of sugars (energy), amino acids (nitrogen), and organic acids (chelation).
- Mucilage Production: Creates a protective "rhizo sheath" for microbes.
- Moisture: Ideal at 60-80% of water holding capacity.
Synergies and Strategic Application
Understanding how different inoculants interact with their environment allows for smarter combinations.
- Bacterial inoculants perform best in well-aerated soils with a redox potential of +250 to +400 mV, moderate temperatures between 25–32°C, and plant roots that have a high density of root hairs, which provide more attachment sites for bacteria.
- Fungal inoculants, including mycorrhiza, prefer slightly lower redox conditions of +200 to +350 mV, cooler temperature ranges of 20–28°C, and plants with extensive lateral root systems, as these roots enhance fungal colonization and symbiotic spread.
- Actinomycetes thrive under highly aerobic conditions with a redox potential of +300 to +450 mV, warmer temperatures of 28–35°C, and rhizospheres characterized by moderate to high root exudation, which supplies the organic compounds they require for sustained activity.
A Practical Optimization Protocol:
- Pre-Application: Assess soil health cards, root health, and native microbial load.
- Application Timing: Apply when soil temperature is >15°C, during active root growth, to moist (not saturated) soil, ideally in early morning or late evening to reduce UV damage.
- Post-Application: Monitor rhizosphere colonization, plant vigour, and soil respiration rates.
The Key Takeaway
Successful biological application is an exercise in system optimization, not a single-factor fix. It requires managing the rhizosphere as a holistic ecosystem where soil physics, chemistry, and biology converge to support plant-microbe performances. The most effective strategy combines regular, detailed soil testing with keen root health assessments, creating a feedback loop for continuous improvement. By tuning the stage—the soil environment—you enable the living actors in your biological products to deliver their full, transformative performance for your crops.
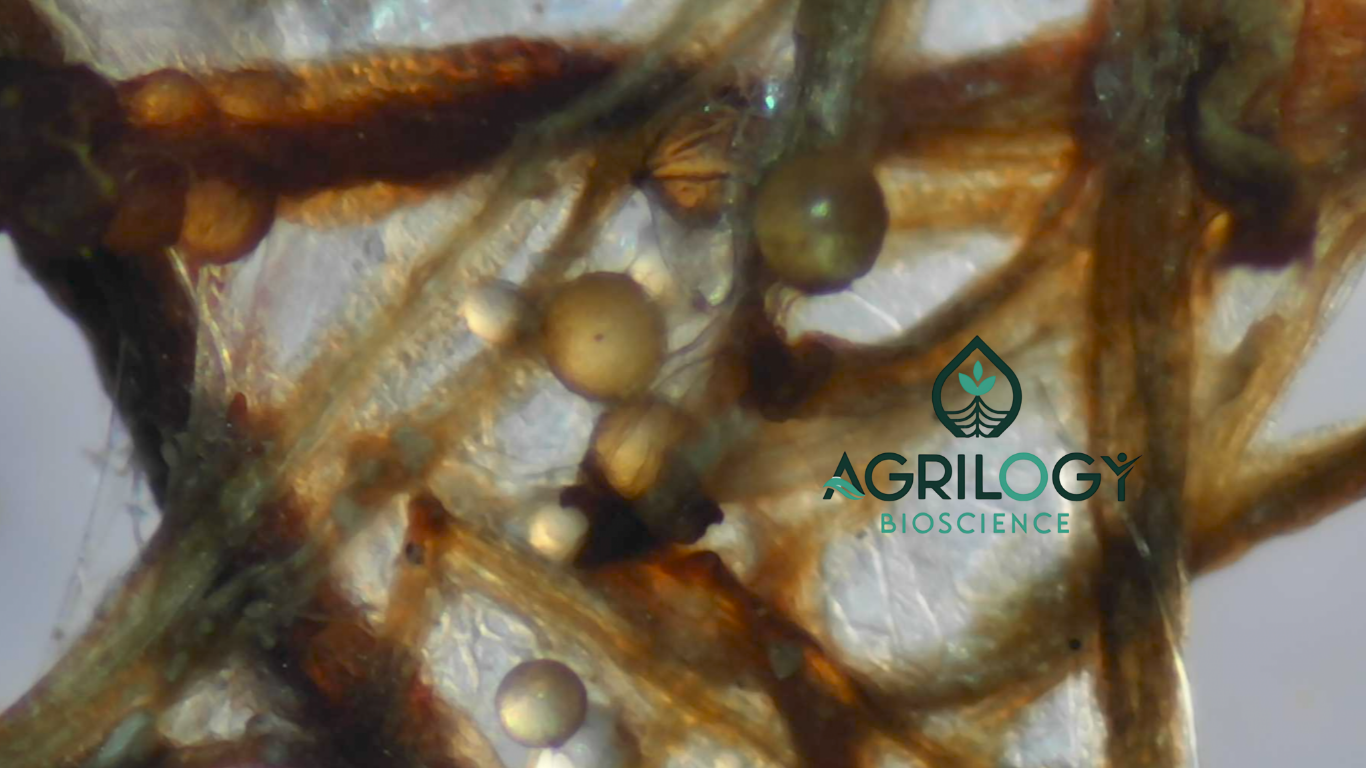
Golden Is Good… But So Are White and Pale Yellow Spores—Here’s the Science
A Story from the Farm That Opened Everyone’s Eyes
A farmer once visited our field demo carrying two samples of mycorrhiza spores.
One had golden-yellow spores—bright, shiny, and visually impressive.
The other had white and pale-yellow spores—simple, dull, easy to underestimate under microscopy.
He asked:
“Saheb, aa banema thi kayu sachu kaam karse? (Which one will work better?)”
Almost everyone pointed to the golden spores.
But the soil told a different story!
When we tested both samples:
- Some golden spores did not stain in the MTT assay → meaning they were dormant or old
- The white and pale-yellow spores turned deep blue hue→ showing strong viability and active metabolism
- Pale spores colonized roots more quickly
- Field plots with pale spores showed better early growth and stronger root networks
The farmer was surprised.
But this is exactly what the science says: Golden spores are good—but white and pale-yellow spores are equally good, often even more active.
Why Colour Alone Misleads Us
A mycorrhiza spore’s colour—whether white, pale yellow, cream, or golden—naturally varies due to its genetics, the host plant it grew with, the soil and environment it developed in, its age, and the carotenoid pigments in its outer wall. Because these factors influence only the outer appearance, colour alone cannot indicate a spore’s internal health or viability. Just like wheat grains that differ slightly in shade but grow equally well, mycorrhiza spores also show harmless colour variation. The real indicator of quality is not colour, but whether the spore is alive and capable of colonizing roots.
White and Pale-Yellow Spores: Naturally Efficient Contributors to Soil Health
White and pale-yellow AMF spores are not weak or immature—multiple authoritative sources, including INVAM (International Culture Collection of Arbuscular Mycorrhizal Fungi) and GINCO-BEL (Belgian Glomeromycota Collection), document that many well-known, highly functional AMF species naturally produce spores in lighter shades. These pale-coloured spores belong to species that play a strong role in nutrient mobilization, hyphal spread, soil aggregation, and root colonization, often performing as effectively as darker or golden spores. AMF reference databases also show clear evidence that pale-coloured spores are common in Glomeraceae and other agriculturally important families, where colour variation is considered a normal taxonomic trait, not a sign of poor quality.
Because of this natural diversity (Polymorphism), pale and white spores consistently contribute to soil ecology by improving phosphorus and micronutrient uptake, enhancing water-use efficiency, increasing soil microbial activity, and supporting carbon storage through extensive hyphal networks.
Thus, the presence of white or pale spores in a product is fully aligned with natural AMF biology and reflects the diversity found in verified, curated AMF collections worldwide.
Myth vs. Reality: Colour Does Not Indicate Viability
It is a common belief that yellow or golden spores are always viable and white or pale spores are non-viable, but MTT assays and global AMF references show this is not true. Golden spores often fail to stain in MTT because they may be senescent, dormant, or pigmented in a way that limits dye penetration, meaning their colour alone cannot confirm activity or viability. In contrast, white and pale-yellow spores frequently stain deep blue, indicating active metabolism, healthy cytoplasm, and readiness to germinate.
So the reality is clear:
Yellow spores are not automatically viable, and white/pale spores are not automatically non-viable. Viability depends on the internal physiology of the spore, not its colour.
What Actually Determines Spore Quality?
Real quality depends on:
- Germination ability
- Cytoplasmic health
- MTT viability
- Hyphal growth
- Root colonization %
- Infection points
- Field performance
Not on colour.
Colour only tells appearance—not power.
Appearance Isn’t Quality: Rethinking AMF Procurement Standards
For procurement teams, judging spore quality by colour alone may seem simple, but it causes significant hidden losses. When white or pale-yellow spores are rejected purely because they don’t “look” ideal, procurement unknowingly discards many viable, high-performing spores that global AMF collections such as INVAM and GINCO-BEL consider completely natural. This leads to avoidable batch rejections, reduced usable output, higher production costs, and inconsistent final product quality.
Colour-based filtering also increases the risk of supplying farmers with visually appealing—but biologically weaker—products. By removing pale spores that often show strong metabolic activity in tests like MTT, procurement unintentionally reduces the overall viability of the formulation. This undermines field performance and long-term trust in the brand.
Moreover, rejecting pale spores deprives the soil of beneficial fungi that contribute to nutrient uptake, root development, and soil structure. These losses highlight why global AMF experts do not use colour as a quality parameter. Procurement accuracy improves dramatically when decisions are based on functional viability, germination, and colonization ability, rather than superficial appearance.
What Should Be Practical Action Check List?
1. Update Procurement SOPs
Adopt the natural colour range of AMF spores — white, cream, pale yellow, and light golden — as acceptable and normal variations.
2. Train QC & Field Teams
Clarify that lighter spores are not weak; they are natural and often show higher metabolic activity in viability tests.
3. Educate Farmers in Simple Terms
Use farmer-friendly explanations such as:
“Spore nu rang quality nathi batavtu; MTT ma neelo rang jivant spore batave chhe.”
4. Follow Science-Based Quality Checks
Rely on MTT viability, germination, and colonization efficiency, rather than colour-based selection.
5. Promote Soil-Health–First Communication
Show how pale spores improve nutrient uptake, root strength, and long-term soil fertility year after year.
Spore Colour Is Just Nature; Viability Is the Truth
Golden spores are good.
White spores are good.
Pale-yellow spores are good.
Every colour is simply a part of nature’s diversity—and all can support strong roots, healthy soil, and better crops. What truly matters is viability, not appearance.
It’s time to move beyond colour myths and build a future driven by science, soil health, and honest quality standards that deliver real value to farmers.
Because ultimately…
Crops don’t grow from the colour of the spore—they grow from the life and activity within it.

Microscope Secrets: Why Light Choice Matters for Spore Identification
Uncovering the true colours of mycorrhiza spores through the science of illumination
Imagine holding the same seed under two different lamps. Under a white LED bulb, it looks pale and plain. But under warm sunlight, the seed suddenly glows with a golden richness.
The same thing happens with mycorrhiza spores under a microscope. The choice of light source can completely change how spores appear—pale and flat in one case, vibrant and mature in another. And this is not just cosmetic; it directly affects how researchers and biofertilizer producers judge spore maturity and viability.
The Story of Light and Spores
Under LED Light
LEDs are like “sharp but picky” torches. They shine narrow wavelengths, mostly cool blue-white. Spores under LED often appear whitish or flat because the pigments in their walls are not fully “woken up.”
Under Halogen Light
Halogen lamps are much closer to natural sunlight. They give a broad, continuous spectrum, rich in warm yellows and reds. Under this light, pigments such as carotenoids and melanin-like compounds come alive, making mature spores glow in yellow, brown, or orange tones.
LED + Halogen Combined
This is the perfect balance—clarity from LED with warmth from halogen. Together, they reveal crisp outlines and true colours, making it easier to judge spore maturity accurately.
Why Do Spores Change Colour?
Mycorrhiza spores are not just “dots”; they are multi-layered capsules:
- Outer wall → Contains natural pigments (yellow, brown, orange).
- Inner wall → Transparent (hyaline).
When only narrow light (like LED) strikes, pigments don’t fully reflect their colours. But when a broad spectrum (like halogen) shines through, the spores display their real, rich tones.
Why This Makes a Difference
- Immature spores (white or hyaline) → Still developing, often less viable, and less effective in helping plants absorb nutrients.
- Mature spores (yellow–brown) → Fully developed, biologically active, and capable of forming strong root associations, resulting in healthier plants and improved yields.
Choosing the right light source ensures that these differences are seen clearly, so researchers and producers don’t mistake weak spores for strong ones.
In a Nutshell
The light source you choose is just as important as the microscope itself. Halogen or a mix of halogen + LED gives the most reliable view of spore maturity. It helps uncover the “true colours” of mycorrhiza spores, supporting better decisions for researchers, biofertilizer companies, and ultimately, the farmers who depend on these invisible allies.
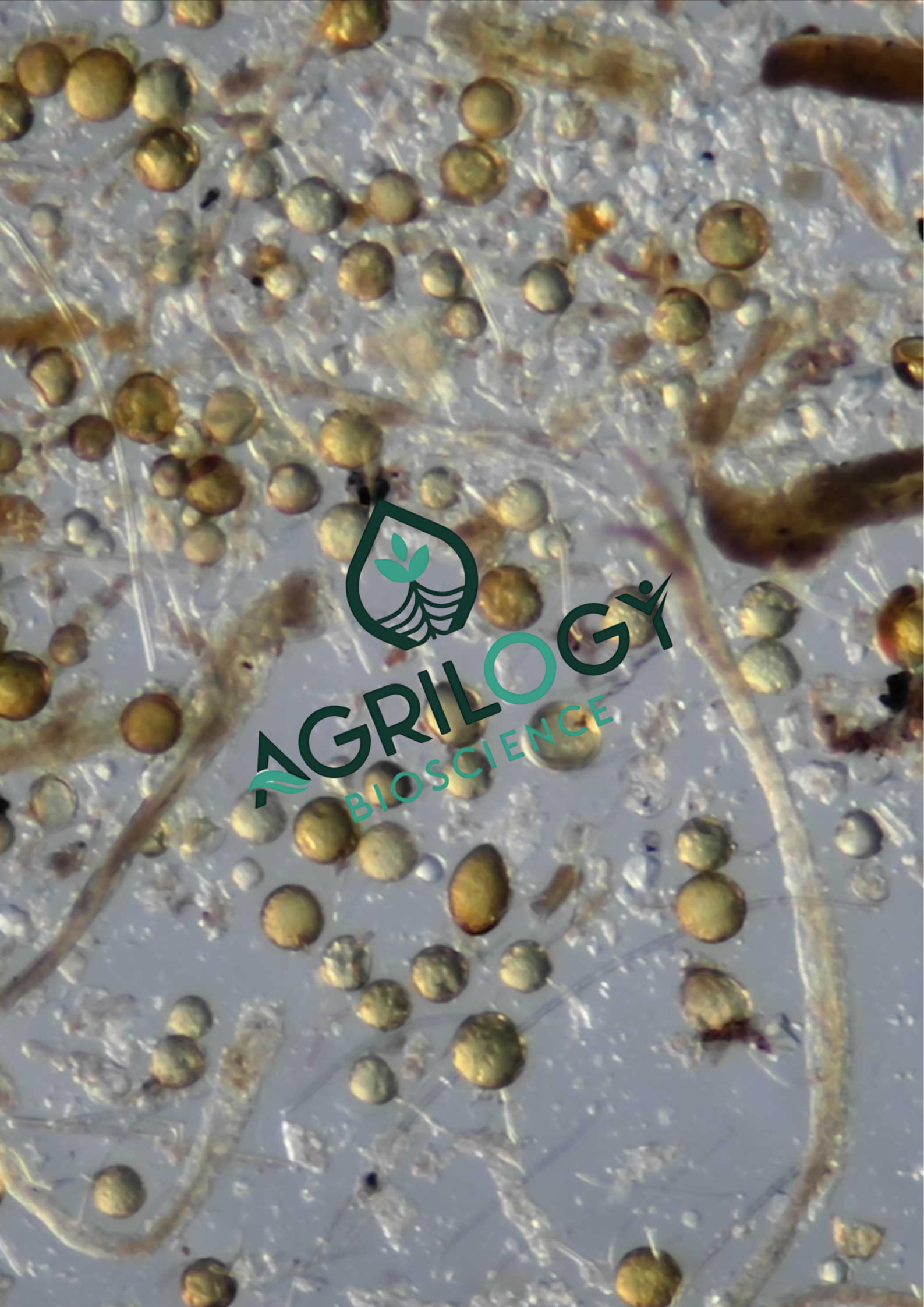
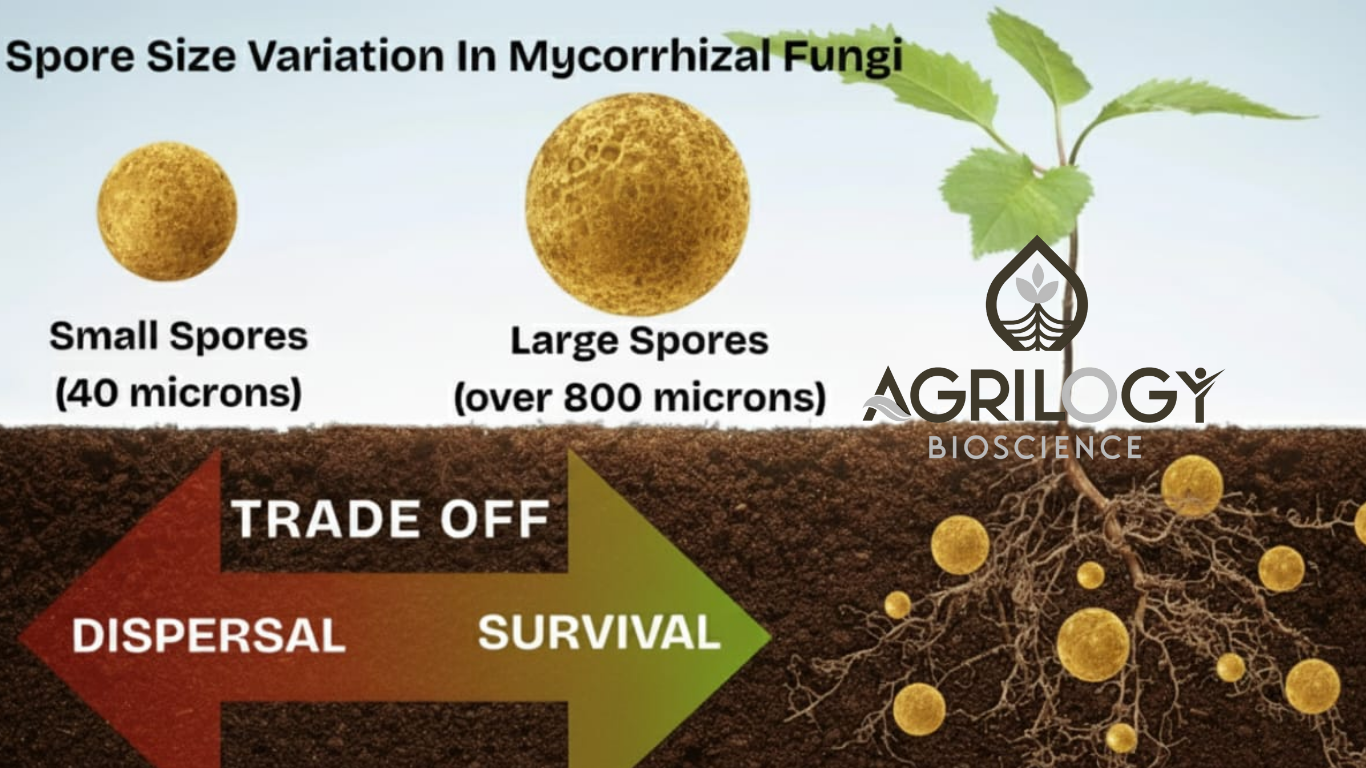
Why Spore Size Matters in Mycorrhizal Fungi ?
The hidden trade-off between survival, dispersal, and ecosystem success
The Basics: What Are We Talking About?
Endomycorrhiza (AMF): These are fungi from the phylum Glomeromycota that live in partnership with the roots of over 80% of land plants.
The Spore: For AMF, spores are the primary survival and dispersal unit. Each spore is a tiny capsule packed with hundreds to thousands of nuclei, lipid reserves, and the potential to start life again by colonizing a p
lant root when conditions allow.
Why Spore Size Matters
Spore size is not random. It’s an evolutionary trade-off between two competing needs:
- Small spores → High numbers, easy dispersal.
- Large spores → Better survival, more reserves.
This balance explains why AMF are so successful in almost every terrestrial ecosystem.
Genetic Blueprint: Different Families, Different Sizes
Each AMF species has a “signature” spore size.
- Acaulospora and Entrophospora → Small to medium spores (40–200 µm).
- Gigaspora and Scutellospora → Giants (300–800 µm, sometimes visible to the naked eye).
The Survival vs. Dispersal Trade-Off
- Large spores
- Pros: Store more food, survive drought or poor soils, grow long germ tubes.
- Cons: Costly to make, fewer in number, poor wind dispersal.
- Small spores
- Pros: Cheap to produce, countless in number, move easily with water, wind, or animals.
- Cons: Less energy stored, short shelf life, need to land very close to a host plant root.
Environmental Drivers
- Nutrient-poor soils: Favor larger spores for survival.
- Disturbed ecosystems (e.g., farms): Favor smaller spores for rapid recolonization.
- Host plant health: Stressed plants may force fungi to produce fewer/smaller spores.
Ecological and Practical Implications
Soil Health Indicator
Shifts in spore size reflect ecosystem status.
- Dominance of small spores → disturbance, intensification, or degradation.
- Large spores → stability, maturity, and resilience.
Agricultural Inoculants
- Small spores: Best for commercial use. They can be mass-produced, flow in irrigation systems, and mix with seed coatings.
- Large spores: Less equipment-friendly but ideal for forestry, orchards, or revegetation projects needing long-term establishment.
Dispersal Across Landscapes
- Small spores: Travel vast distances via wind and water (even between continents).
- Large spores: Spread locally, often hitchhiking with earthworms, rodents, or other soil movers.
Fascinating Twist: Spore Clusters
Some AMF species combine both strategies by producing spore clusters (sporocarps).
- Example: Glomus species form clusters of many small/medium spores.
- Advantage: Protection plus the ability to disperse multiple spores at once via animals or disturbance.
The Bigger Picture: r- vs K-Strategies
In ecological terms:
- Large spores = K-selected strategy → “quality over quantity.”
- Small spores = r-selected strategy → “quantity over quality.”
This simple size difference drives the resilience and spread of one of Earth’s most important plant-fungal partnerships.
The Bottom Line
Spore size is not just a microscopic detail—it’s a survival strategy. It shapes how fungi spread, endure harsh conditions, and support the plants we rely on for food, forests, and healthy ecosystems.
Nature’s Design for Growth: Diverse Roots, Resilient Plants
Nature’s Original Strategy: Why Plants Never Grow Alone?
Walk through any forest, grassland, or uncultivated patch of earth, and you’ll witness a marvel of natural engineering—not above the ground, but beneath it.
In natural ecosystems, plants never form relationships with just one species of mycorrhizal fungi. Instead, they depend on a rich, diverse community of fungal allies living in the rhizosphere (the zone of soil surrounding their roots). This isn’t just biological event —it’s an evolved survival strategy over fine-tuned hundreds of millions of years.
This underground symbiotic network, called a mycorrhizal consortium, allows plants to access nutrients, moisture, and microbial defense mechanisms far beyond their reach alone. Each fungal species contributes its own strengths, allowing plants to adapt to changing conditions, combat stressors, and thrive in unpredictable environments.
So if nature—untamed and unmodified—thrives on microbial diversity, why should modern agriculture rely on just one species of mycorrhiza in its inoculants?
At Agrilogy Bioscience, we believe the best agricultural innovations come from studying and replicating nature's own solutions. That’s why our Agright™ VAM product is designed as a multi-species consortium, echoing the very strategy nature uses to empower plant life.
Let’s explore why this natural model of diversity is not only more effective—but essential for resilient, sustainable farming.
Where the Single-Species Model Fails?
Commercial inoculants often rely on just one fungal species—usually Glomus intraradices (Rhizophagus irregularis)—known for its capacity to improve phosphorus uptake. However, this monoculture approach suffers from serious limitations:
- Narrow Functional Scope:
A single fungal species can only contribute one set of benefits—often limited to one nutrient or function. This oversimplifies the plant’s needs and risks leaving it unsupported in times of stress or transition.
- Soil Sensitivity
In high-phosphorus soils (common in agriculture), G. intraradices often goes dormant. That means no support to the plant—no matter what the label promises.
- Uniform Colonization
Root systems and their development vary dramatically over time. A single mycorrhizal species can’t effectively colonize all parts of the root or adjust to different plant growth stages.
Mycorrhizal Consortia: Nature’s Blueprint for Resilience
In natural settings, plant roots are colonized by multiple mycorrhizal species simultaneously. This strategy provides:
- Biological redundancy: If one species falter, others compensate.
- Functional diversity: Different fungi help with different nutrients, resist different pathogens, and handle different stressors.
- Lifecycle flexibility: Some fungi activate early in plant development; others offer long-term benefits.
Inspired by this model, our Agright™ VAM features a carefully selected four-species blend, each with a unique role to play. Together, they create a resilient symbiotic network, tailored to real-world conditions and diverse crop needs.
The Power of the Agright™ VAM Consortium—Expanded Benefits
- Functional Redundancy & Synergy
Each fungal species brings distinct capabilities. Whether it’s phosphorus solubilization, nitrogen uptake, drought mitigation, or pathogen suppression, consortia provide a full spectrum of support—allowing plants to "choose" the partner they need, when they need it.
- Resilience Under Stress
Environmental fluctuations are inevitable. Some fungi in the consortium may become dormant under specific conditions (like high phosphorus), but others remain active—ensuring your crop is never without microbial allies.
- Accelerated and Diversified Colonization
The speed and pattern of root colonization differ among fungi. Early colonizers initiate quick support during germination or transplant shock, while others form deep, stable relationships that enhance long-term performance.
- Full Lifecycle Coverage
From seedling to flowering and harvest, a plant's needs evolve. With a diverse fungal network in place, your crop receives tailored support through every stage of its growth.
Nature Doesn’t Do Monoculture—Why Should You?
Every inch of living soil in the wild is flourishing with fungal diversity. Nature understands that no single organism can do it all. This complex underground system ensures plant survival, adaptability, and productivity.
By emulating this model through multi-species mycorrhizal consortia, we not only improve plant performance but also regenerate soil health, reduce dependence on chemical inputs, and move toward true agricultural sustainability.
A Smart Investment for Future-Ready Farming
Choosing the right inoculant isn’t just a technical matter—it’s a strategic decision. Single-species products may offer narrow, short-term benefits, but they fall short in the dynamic and often stressful environments modern growers face.
By investing in a mycorrhizal consortium like Agright™ VAM, you provide your crops with:
- Adaptive microbial support
- Broader nutrient access
- Greater resistance to environmental stress
- Faster establishment and long-term vitality

Nature Knows Best: Trust the Underground Network
Nature has already done the research. It chose diversity. It chose collaboration. It chose resilience through complexity.
At Agrilogy Bioscience, we bring these natural principles into your fields—helping you grow smarter, stronger, and more sustainably.
The Complexity Behind MTT Staining: Navigating Variability in Spore Viability Testing
When it comes to assess the viability of fungal spores (especially VAM Fungi), the MTT assay seems like a simple solution—just add the dye and observe the colour change. But what happens when the colour reactions are anything but straightforward? Dive into the controversies and complexities behind MTT staining and discover why accurate interpretation of spore viability is trickier than it seems.
Assessing the viability of fungal spores, particularly those from arbuscular mycorrhizal fungi (AMF or VAM), is essential for understanding their role in plant symbiosis.
One widely used method for evaluating spore viability is the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide) assay. This assay measures the metabolic activity of spores, offering a visually accessible indicator of their health. However, despite its popularity, MTT staining can lead to inconsistent and sometimes controversial interpretations. Let’s break down the principles behind MTT, its colour interpretations, and the factors that cause variability in results.
What is MTT Dye?
MTT is a yellow tetrazolium dye that is reduced by cellular dehydrogenases, enzymes that play a critical role in cellular respiration. When the dye is reduced in respiring cells, it forms a purple formazan product. The intensity of the purple colour directly correlates with the metabolic activity of the cells or spores, making MTT a useful tool for assessing cell viability. For VAM spores, this process indicates whether the spores are metabolically active and potentially viable for symbiosis with plant roots.
How Does MTT Work?
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) staining is a widely used assay to assess cell viability based on metabolic activity. When introduced to living AMF or VAM cells, MTT is reduced by mitochondrial dehydrogenase enzymes, which disrupt the tetrazolium ring structure and form a purple-coloured formazan product. This reaction occurs in metabolically active cells where enzymes, aided by cofactors like NADH or NADPH, facilitate the reduction process.
The resulting formazan accumulates inside the cytoplasm, and the intensity of the colour produced correlates directly with the cellular metabolic activity of the spore. The ease with which MTT passes through the lipid membranes of viable cells ensures its effective uptake, making it an excellent indicator of cell health and viability. The amount of formazan formed is a quantitative measure, offering a reliable insight into the viability of cells or spores, such as VAM spores, by highlighting their metabolic capacity.
MTT Assay Protocol for Spore Viability Assessment:
The MTT Assay Protocol involves weighing 1 gram of the sample (e.g., fungal spores) and washing it under tap water multiple times. The collected spores are diluted in freshly prepared 0.25% MTT dye. The mixture is then inverted to ensure thorough mixing and incubated at 27°C in the dark for 24, 48, and 72 hours. After incubation, the sample is washed 3-4 times with tap water and then suspended in 10 ml of RO water. A 0.5 ml aliquot is placed on a petri dish and examined under a stereo zoom microscope for spore viability.
Colour Interpretation and Its Significance:
The colour changes in MTT staining offer critical insights into spore viability. However, these changes are not always straightforward:
- Red/Pink/Purple Spores: Viable spores typically stain red, pink and purple with the intensity of the colour corresponding to the level of metabolic activity. A purple colour indicates active dehydrogenase enzymes, while a pink hue suggests lower metabolic activity.
- Black Spores: Black spores are often assumed to be non-viable, as they suggest excessive formazan accumulation in the cytoplasm upon reduction by cellular reducing agents. However, black coloration may also result from overstaining, leading to ambiguities in viability interpretation.
- Unstained Spores: Chemically killed or non-metabolically active spores will not reduce MTT, and thus remain yellow, indicating non-viability.
Despite these general rules, variability in results often complicates the interpretation of spore viability.
Controversies and Sources of Variability in MTT Staining:
Although MTT staining is widely used, its application comes with notable challenges. Here are some key factors that contribute to its variability:
1. Overstaining and Misleading Black Spores:
✓Black coloration in MTT staining is commonly linked to non-viability, but this can be misleading. Excessive MTT accumulation or prolonged incubation can result in black spores, even if they are still viable. Therefore, black coloration should not be automatically interpreted as a sign of non-viability.
2. Impact of Spore Age and Wall Thickness:
✓Older spores, particularly those that have been stored for extended periods, require higher concentrations of MTT or longer incubation times to reach a maximum colour change. Furthermore, the thickness of the spore wall can affect the penetration of MTT. Thicker walls may limit the dye’s access to the enzymatic sites, resulting in inconsistent staining and variability in colour reaction.
3. Dormant Spores and Metabolic Decline:
✓Spores stored for long periods often experience a decline in metabolic activity. These dormant spores may still reduce MTT, producing faint red or pink hues despite being non-viable in terms of germination or colonization. This presents a challenge in distinguishing between dormant and truly viable spores, as they may still display metabolic activity despite not being capable of establishing symbiosis.
4. Chemical Reducing Agents in Non-Viable Spores:
✓Non-viable spores may still reduce MTT due to the presence of reducing agents like cysteine, sulphur-containing compounds, or reducing sugars. These chemicals can catalyse the reduction of MTT even in the absence of active dehydrogenase enzymes, leading to false positives and incorrect conclusions about spore viability.
5. Species-Specific Variability:
✓Different VAM species exhibit varying metabolic activity levels. As a result, their responses to MTT can differ significantly. Some species may require longer incubation or higher concentrations of MTT to achieve a visible colour change, making standardization across species challenging.
6. Difficulties in Interpreting Pink Spores:
✓Pink spores can be particularly problematic. A faint pink colour may suggest minimal metabolic activity, but it is difficult to determine whether the spores are dormant or in a state of partial viability. These spores may be capable of reducing MTT but lack the resources to germinate or colonize plant roots.
Conclusion: Navigating the Complexities of MTT Staining:
While MTT staining is a useful and widely employed technique for assessing the viability of fungal spores, it is far from perfect. A thorough understanding of its limitations is essential for accurate interpretation. Factors such as overstaining, spore wall thickness, spore age, and the presence of reducing agents must be carefully considered when analysing the results. In particular, the ambiguous interpretation of black or pink spores underscores the need for caution in drawing conclusions based solely on MTT staining.
To minimize variability and ensure reliable results, optimization of experimental conditions is essential. Researchers must account for species-specific characteristics, spore age, and other confounding factors in order to achieve accurate assessments of fungal spore viability. In summary, MTT staining is a valuable tool, but its complexity requires a refined approach for obtaining meaningful data.
Key Takeaways:
- MTT staining helps assess spore viability by measuring metabolic activity through colour changes.
- Red or pink colouration typically indicates viable, respiring spores, while black colouration can indicate overstaining or non-viability.
- Factors like spore age, wall thickness, and the presence of reducing agents can affect MTT staining results, leading to potential misinterpretations.
- Optimization of experimental conditions is crucial for accurate viability assessment across different VAM species.

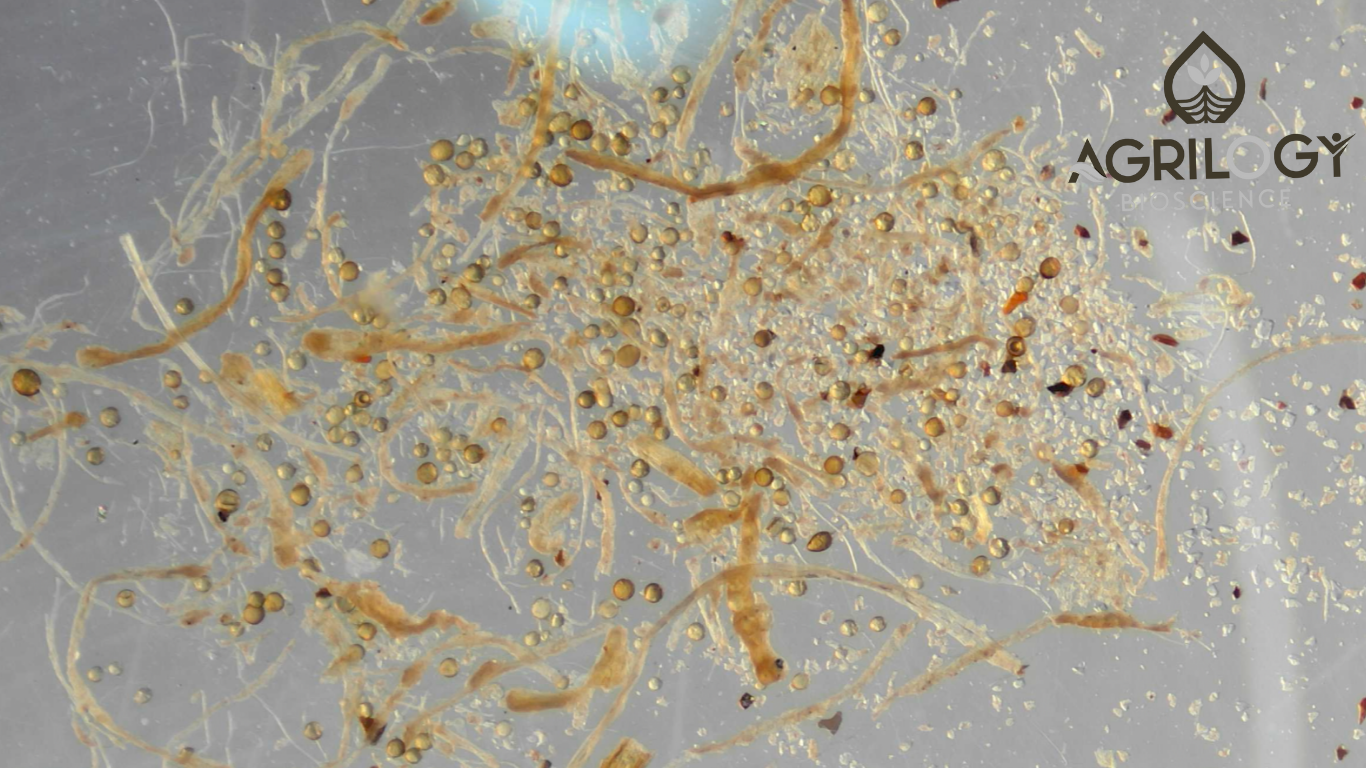
Mastering Spore Counting: A Comprehensive Guide for Accurate VAM In Solid (Powder or Granular) Formulation Testing
When it comes to evaluating the effectiveness of Vesicular Arbuscular Mycorrhizal (VAM) spores in powder or granular formulations containing humic substances, seaweed extracts, and inert carriers like bentonite or water-soluble dextrose, proteins etc.; spore counting is the key to ensure product quality. But what happens when companies make claims like 25,000 spores per gram, and the reality doesn’t quite match up? How do we ensure these claims hold true? The answer lies in precision in spore counting through Stereo Zoom Microscopy, a tool that offers 3D views and enhanced resolution to see spores in finer detail than traditional methods.
In this blog, we will walk you through Agrilogy Bioscience Private Limited’s Spore Evaluation Method, an established and tested protocol, alongside the best practices for counting spores in VAM formulations with challenging carriers, such as humic substances and bentonite. We’ll discuss the methodology, tools, and tips to make your spore evaluations accurate, reliable, and efficient.
Why Spore Counting Matters: Accuracy is Key
Accurate spore counts are a critical part of VAM quality control (QC). While many companies claim a high spore count (like 25,000 or even 1,00,000 spores per gram), the actual spore count often tells a different story when examined under the microscope. Ensuring precision in spore counting can prevent discrepancies between claimed and actual spore concentrations, thereby fostering trust and consistency. This is where Stereo Zoom Microscopy comes in. Offering superior resolution and a 3D view, it allows for a clearer picture of spore morphotypes compared to conventional compound microscopes. But it’s not just about the technology; the method you use plays a massive role in accuracy.
Agrilogy Bioscience Private Limited’s Spore Evaluation Method: The Essentials
Let’s dive into the methodology that Agrilogy uses for spore evaluation. This method is designed to ensure precise spore counting by following a step-by-step protocol. The process relies on careful sample preparation and the use of specialized equipment to achieve the most accurate results possible.
Tools Required:
- Sugar Tubes
- 100 ml Glass Measuring Cylinder
- Micropipette & Tips (1000 μl)
- Metler for precise weighing
- Vortex Machine
- Stereo Zoom Microscope
- Petri Plates (Grided or Non Grided)
- Scissors & Tissue Paper
How to Evaluate Spores in Different Carrier Types: Two Reliable Lab Methods
Accurately evaluating spore concentration is essential for quality control in VAM formulations. Depending on the type of carrier used—whether water-soluble or more complex solid carriers—different lab techniques are needed to extract and count spores effectively.
1. Spore Evaluation in Water-Soluble Carriers:
This method is ideal for products where the carrier dissolves easily in water, such as powders formulated with sugars or similar materials.
Steps:
- Weigh a small, precise amount of sample (10–100 mg), depending on the expected spore load.
- Mix the sample with 20 mL of distilled water and vortex gently (around 2000 rpm) to disperse spores without damaging them.
- Using a micropipette with a cut tip, transfer 1 mL of the mixture dropwise onto a sterile Petri plate.
- Rinse the pipette tip with clean water using a new, uncut tip and add this to the same plate.
- Observe the sample under a stereo zoom microscope (1X–4.5X magnification) and count the spores.
- Calculate the spore concentration per gram using the dilution factor and sample weight.
This method is quick, straightforward, and well-suited for clean, soluble formulations.
2. Spore Evaluation in Solid or Insoluble Carriers:
For products with humic substances, peat, or insoluble binders, spores are often trapped in debris, requiring a more physical separation process.
Steps:
- Mix 0.1–5 grams of the sample with 100 mL of water, depending on spore density and carrier type.
- Decant the mixture through a two-sieve system: a 250 μm sieve to retain large debris and a 37 μm sieve to collect spores.
- Rinse the sieves 4–5 times with water to wash away humic matter or insoluble particles that may coat spores.
- Backwash the fine sieve using wash bottles to collect the spores into a 20 mL tube.
- Transfer 1 mL of this sievate to a Petri plate and observe under a stereo zoom microscope.
- As before, calculate the final spore count per gram using your observed count and dilution factor.
This method is more involved but necessary when dealing with particulate or organic-rich carriers.
Both methods offer reliable ways to assess spore viability and concentration, with each tailored to specific types of carrier systems. Choosing the right protocol helps ensure accurate measurements, supporting product quality, regulatory compliance, and scientific research.
Do's and Don'ts for Accurate Spore Testing
Here are some critical Do’s and Don’ts to follow during the spore testing process:
Do’s:
- Use Accurate Weighing: Ensure the sample weight is precise with a tolerance of ± 0.0005 mg. Opt for minimum of 100 mg of sample to overcome overlapping issues and avoiding variation factor ensuring accurate estimation of spore quantity.
- Vortex Gently: Vortex the sample at low speed for 2-3 minutes to avoid damaging the spores.
- Mount Carefully: When transferring droplets to the Petri plate, ensure that the drops don’t spread to the edges or overlap.
- Multiple Readings: Always take 5-10 readings and calculate the average to minimize variability.
- Sample Preparation: Sugar tubes or test tubes should be used instead of beakers. This helps minimize false negatives caused by spores sticking to the container’s surface, and the reduced surface area of tubes allows for better mixing, which is key for accurate evaluation.
- Dilution Factor: The sample should be diluted with a maximum of 20 ml to manage the high concentration of spores and to reduce the likelihood of false positives.
Don’ts:
- Avoid High-Speed Vortexing: High speeds can shear spores, resulting in inaccurate counts.
- Do Not Skip the Washing Step: Carefully wash the micropipette tip to remove any leftover spores.
- Examine Only Intact Spores: Do not count broken, cracked, or transparent spores.
- Sieve Thoroughly: If you notice excessive debris, sieve the sample again through 37 μm mesh before re analyzing.
Conclusion: Ensuring Accurate Spore Counts for Quality Assurance

Accurate spore counting is crucial to maintain the integrity and effectiveness of VAM and granular formulations. By following the meticulous methodologies outlined here, you can ensure precise spore evaluations, regardless of the challenges posed by organic carriers or inert granules. Using Stereo Zoom Microscopy for observation, paying attention to the details of sample preparation, and adhering to best practices will lead to more accurate assessments, boosting the reliability of your products and fostering trust in your brand.
If you have any further questions or would like to share your experiences with spore testing, feel free to reach us.
ESTIMATING THE EFFECTIVENESS OF INFECTIOUS PROPAGULES (IP) USING MPN METHOD
Estimating the concentration of viable mycorrhizal propagules in a sample is a crucial step in assessing the effectiveness of biofertilizers and soil inoculants. In this post, we break down the step-by-step process of calculating the Most Probable Number (MPN) of infective propagules using dilution series and replicate tube assays—making a complex method simple and approachable for researchers, students, and industry professionals alike.
What is the MPN Method?
The Most Probable Number (MPN) method is a statistical technique used to estimate the number of viable microorganisms—such as infective propagules (IP) of mycorrhizal fungi—in a given sample. It relies on a series of dilutions and observations of microbial growth (or colonization) in multiple replicates to calculate a probable concentration in the original sample.
Experimental Setup
In this case, four serial dilutions of a sample (10⁻¹, 10⁻², 10⁻³, and 10⁻⁴) were tested. Each dilution was replicated in 5 tubes to observe mycorrhizal colonization. The number of tubes showing positive colonization at each dilution was recorded as follows:
- 10⁻¹: 5/5 tubes positive
- 10⁻²: 5/5 tubes positive
- 10⁻³: 3/5 tubes positive
- 10⁻⁴: 2/5 tubes positive
Identifying the MPN Index
To calculate the MPN, you only need the results from the last three consecutive dilutions that show a decreasing number of positive tubes:
- P1 = 5 (from 10⁻² dilution)
- P2 = 3 (from 10⁻³ dilution)
- P3 = 2 (from 10⁻⁴ dilution)
This gives an MPN index of 5-3-2.
Using the MPN Table
Next, consult a standard 5-tube MPN table to find the most probable number corresponding to the 5-3-2 combination. According to the MPN table, the value is:
➡️ 14
This number represents the most probable number of infective propagules in the dilution level of P2.
Calculating Total IP/g of Substrate
Now, use the following formula to calculate the total number of infective propagules per gram of substrate:
Total IP/g=MPN value×Dilution factor of P2/Dry mass of sample (g)
Given:
- MPN value = 14
- Dilution factor of P2 (10⁻³) = 1000
- Dry mass of product sample = 10 g
Total IP/g=14×1000/10=1400 IP/g
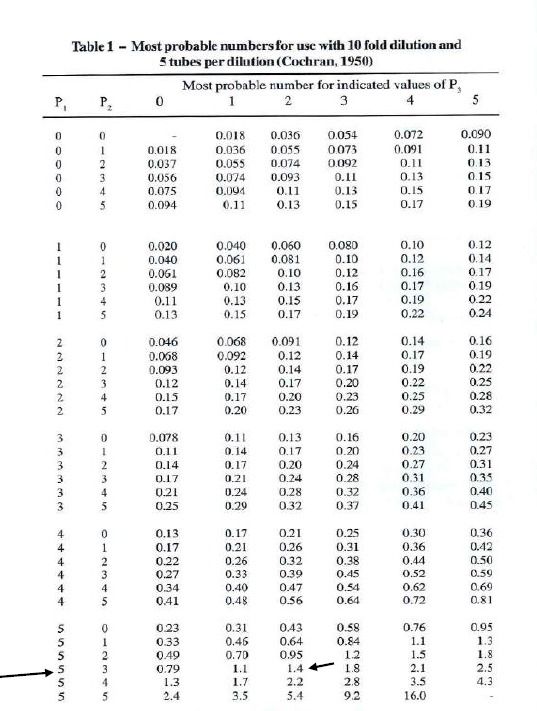
Conclusion
The MPN method provides a statistically sound and practical way to estimate infective propagules in soil or inoculant samples. In this example, the sample contains 1400 infective propagules per gram—a critical metric for evaluating biofertilizer quality and microbial inoculant potency.
ASSESSMENT OF EFFECTIVENESS OF MYCORRHIZAL INOCULANT IN SORGHUM BY IN VIVO POT CULTURE METHOD
Sorghum is a climate resilient crop known for its drought tolerance and nutritional benefits in arid regions, but even the challenges of plants can be benefitted from a little help. Enter Vesicular Arbuscular Mycorrhiza (VAM), a beneficial fungus that plays a critical role in improving plant health, especially under challenging growing conditions (biotic and abiotic stresses). VAM serves number of benefits to the plant health but how do we know if our VAM inoculum is truly effective?
In this blog, we’ll dive into how VAM inoculation can boost sorghum growth and how researchers are assessing the effectiveness of these fungal partnerships through a traditional in vivo pot culture method.
The Symbiosis of VAM: How Fungi and Plants Thrive Together
Plants and fungi have formed a unique partnership over millions of years. VAM fungi penetrate cortical region of the plant roots, creating a vast network of fungal mycelia (intraradical hyphae) that increases the plant's extent to nutrient availability zone through increase in surface area of mycelia. This relationship allows the plant to access nutrients like phosphorus, nitrogen, zinc etc. which are often unavailable in the plant`s reach zone in the rhizosphere, while the plant provides the fungi with essential sugars and lipids.
For crops like sorghum, this partnership can significantly improve plant growth, especially in nutrient-deficient or water and heat stressed environments.
Assessing the Inoculum Potential: The In Vivo Pot Culture Method
To evaluate the effectiveness of mycorrhizal inoculum, researchers employ an in vivo substrate based pot culture method. This technique involves growing plants in suitable substrate where the inoculated fungi can be monitored for root colonization and overall plant growth for 45-60 days.
Here’s a quick look at how it works:
- Preparation of the Inoculum: Mix the VAM spores with a sterilized substrate (sand, soil, and cocopeat). Serial dilutions (10-1 to 10-4) of the inoculum are created to test the effect of different concentrations of VAM on plant growth.
- Planting Sorghum Seeds: After preparing the inoculum, sorghum seeds are sown into containers filled with the substrate mixture. Each container represents a different dilution, allowing us to test the effectiveness of various inoculum concentrations.
- Observing Growth and Root Colonization: After germination, only one plant per container is maintained, and the plant grows for 45 days. Researchers then examine the roots for signs of mycorrhizal colonization using a microscope. The presence or absence of mycorrhizae is noted, and the data is used to calculate the inoculum potential (IP) of the fungal sample.
- Calculating Inoculum Potential (IP): Using the Most Probable Number (MPN) method, researchers can estimate the inoculum potential—a measure of how many viable fungal propagules (spores) are present per gram of substrate. This helps determine the optimal level of inoculum for maximum plant growth.
The Benefits of VAM Inoculation for Sorghum
The results of these experiments often reveal significant benefits for sorghum growth, especially in challenging environments. Here’s why inoculating with VAM fungi can be a game-changer for sorghum farmers:
- Stronger Root Systems: VAM inoculation results in more extensive root networks, allowing plants to absorb nutrients more efficiently, especially phosphorus.
- Better Growth and Yield: VAM-treated plants typically exhibit increased height, weight, and biomass. In some studies, the inoculation has also led to higher yields of grain.
- Improved Stress Resistance: With increasing global challenges like climate change and soil degradation, VAM’s role in enhancing drought and salinity tolerance is critical. Sorghum plants inoculated with VAM show better survival rates and more robust growth under such stress conditions.
Challenges and Future Directions
While VAM inoculation in in vivo technology offers many advantages, the process isn’t without challenges. Standardizing spore quality, preventing contamination, and ensuring uniformity in the fungal inoculum can be difficult. However, these obstacles are not insurmountable. Ongoing research is focused on refining inoculum production methods and developing more efficient ways to apply VAM fungi in field settings.
Conclusion: A Symbiotic Solution for the Future of Sorghum Farming
Inoculating sorghum seeds with Vesicular Arbuscular Mycorrhiza (VAM) is proving to be a powerful tool for improving plant growth, resilience, and overall crop yield. By using techniques like in vivo pot culture, growers are better able to assess the effectiveness of VAM inoculum and refine these methods for large-scale farming.
Whether you're a researcher, farmer, or simply a plant enthusiast, understanding the power of beneficial fungi like VAM can help us develop more sustainable and productive agricultural practices. As we continue to explore the full potential of these mycorrhizal partnerships, the future of crops like sorghum looks brighter than ever.
We will learn about MPN estimation for IP analysis in our next blog. Stay connected!

Give Your Crops the Foundation They Need to Thrive!
From drought resistance to enhanced nutrient uptake, find out how Agrilogy Bioscience Private Limited’s mycorrhizal spores can set your plants up for success.
When we think of healthy crops, we often picture what we can see—lush green leaves, strong stems, and bountiful harvests. But what if the real key to plant health lies underground?
Beneath the surface, an ancient and powerful alliance is at work. This partnership between plants and mycorrhizal fungi is transforming how we grow, nourish, and sustain our crops.
In this post, we’ll explore how different species of mycorrhizal fungi are becoming the unsung heroes of modern farming—and why now is the time to pay attention to what’s happening beneath our feet.
At Agrilogy Bioscience Private Limited, we believe in the power of nature to transform the way we grow crops. Mycorrhizal fungi, through their symbiotic relationship with plant roots, offer unparalleled benefits to your soil and plants. Whether you’re working in nutrient-poor, acidic, or drought-prone soils, our diverse range of mycorrhizal spores helps improve soil structure, enhance nutrient uptake, and increase plant resilience. Dive into the world of mycorrhizal fungi with Agrilogy Bioscience Private Limited’s premium spore offerings designed to help your crops thrive.
The Hidden Partners Beneath Every Strong Crop
The first time I witnessed the power of mycorrhizal fungi, it was a side-by-side field trial. Both fields had the same crop, but only one was treated with Rhizophagus intraradices, a widely studied arbuscular mycorrhizal species.
The difference was staggering.
The treated plants showed better growth, richer leaf color, and—most notably—resilience in the face of reduced irrigation. How? Because R. intraradices had developed an intricate network of hyphae (fine fungal threads) that extended the plant’s root system, allowing it to draw nutrients and water from soil zones far beyond the reach of natural roots.
And it wasn’t just that one species making a difference:
- Funneliformis mosseae showed remarkable efficiency in acidic, nutrient-depleted soils. Its ability to mobilize phosphorus helped revive crop performance in previously underproductive land.
- Claroideoglomus etunicatum played a vital role in areas hit by drought, strengthening root systems and supporting water retention.
These invisible organisms are not just supporting plant growth—they’re reshaping what’s possible in agriculture.
Rebuilding Soils with Biology, Not Just Chemistry
In many regions around the world, soils have been exhausted by years of monoculture, over-fertilization, and erosion. The usual response? Chemical inputs. But what if we could rebuild soil naturally?
That’s where Rhizophagus fasciculatum comes in—a fast-colonizing mycorrhizal fungus ideal for disturbed or degraded land.
In one example, a field worn out from continuous cropping showed dramatic recovery after inoculation with R. fasciculatum. The results were impressive:
- Soil porosity improved, enhancing oxygen exchange and root penetration
- Fertilizer inputs were reduced by up to 30%
- Plants developed deeper and healthier root systems
The fungus didn’t just feed the crop—it restructured the soil itself.
Mycorrhizal fungi serve as soil engineers. They stabilize aggregates, improve water retention, and help cycle nutrients organically. In arid regions or post-clearance restoration efforts, their value is immeasurable.
Tailored Fungal Solutions for Diverse Challenges
What makes mycorrhizal fungi so powerful is their diversity. Different species thrive in different conditions—and choosing the right one can transform your soil management strategy.
At Agrilogy Bioscience Private Limited, we’re proud to offer a range of high-quality, highly effective mycorrhizal spore species, each carefully selected to meet your specific agricultural needs. Here’s a snapshot of a few notable species and their unique strengths:
Meet Agrilogy Bioscience Private Limited’s Top Mycorrhizal Species

Every farm has unique needs, and we believe in offering the right solution for your soil, climate, and crops. Whether you're farming in nutrient-depleted soils, working to restore degraded land, or looking to boost plant growth in drought-prone areas, we have a species of mycorrhizal fungi that can meet those needs. Let’s explore the diverse and valuable species we offer:
1) Rhizophagus intraradices (Glomus intraradices)
- Spore Size: 40–140 μm
- Colour: Pale yellow to light brown
- Best for: Agricultural soils with varying pH, crops requiring high phosphorus uptake, and drought-prone conditions.
- Special Characteristics: Known for its efficiency in improving phosphorus uptake, R. intraradices also enhances plant drought tolerance and helps improve soil structure. Its rapid colonization and extensive hyphal networks contribute to plant growth even in challenging environments, making it an excellent choice for a wide variety of crops.
- Why Choose This Species? Perfect for crops in agricultural soils where enhancing phosphorus uptake and drought resilience is key.
2) Funneliformis mosseae (Glomus mosseae)
- Spore Size: 120–200 μm
- Colour: Yellow-brown to dark brown
- Best for: Acidic soils, nutrient-depleted lands, and crops that need a boost in phosphorus uptake.
- Special Characteristics: F. mosseae is extremely effective in acidic soils, where other forms of mycorrhizal fungi may struggle. Its ability to absorb phosphorus efficiently helps restore nutrients in soils that have been depleted over time. It also supports plant growth in environments where nutrient availability is limited.
- Why Choose This Species? Ideal for restoring soils that have become nutrient-depleted, especially in acidic conditions.
3) Rhizophagus fasciculatum (Glomus fasciculatum)
- Spore Size: 60–110 μm
- Colour: Pale yellow to yellow-brown
- Best for: Fast colonization in disturbed soils, agricultural soils, and arid regions.
- Special Characteristics: R. fasciculatum is known for its rapid colonization capabilities, making it a great choice for disturbed or degraded soils. It helps speed up the recovery process in soils that have been damaged by overuse or environmental stress.
- Why Choose This Species? Perfect for quick soil restoration in disturbed environments, arid areas, or recently cleared lands.
4) Rhizophagus proliferum (Glomus proliferum)
- Spore Size: 70–110 μm
- Colour: Pale yellow (hyaline)
- Best for: Forest soils, disturbed agricultural lands, and regions requiring soil restoration.
- Special Characteristics: R. proliferum is a rapid colonizer that establishes quickly in disturbed soils, making it essential for soil restoration projects. It is also beneficial for enhancing root biomass, especially under saline conditions.
- Why Choose This Species? Excellent for rapidly restoring degraded lands or in situations where fast plant establishment is needed.
5) Rhizophagus clarus (Glomus clarus)
- Spore Size: 100–120 μm
- Colour: White to pale yellow
- Best for: Agricultural systems with sandy loam soils, nutrient cycling, and early-stage plant growth.
- Special Characteristics: R. clarus is known for its ability to form resilient spores that thrive in low-nutrient soils. It helps improve soil structure, supports nutrient cycling, and enhances early plant growth, making it a go-to choice for sandy soils and disturbed ecosystems.
- Why Choose This Species? Perfect for improving soil health and supporting early plant growth in sandy and nutrient-poor soils.
6) Claroideoglomus etunicatum (Glomus etunicatum)
- Spore Size: 80–150 μm
- Colour: Pale yellow to red-brown
- Best for: Agricultural fields, disturbed ecosystems, and areas prone to drought.
- Special Characteristics: C. etunicatum is highly resilient, tolerating both environmental stresses and pathogens. It improves root growth, boosts plant resilience, and is particularly effective in areas with water scarcity or low soil fertility.
- Why Choose This Species? Ideal for growing crops in drought-prone or stressed environments where rapid root growth is necessary.
Why Choose Agrilogy Bioscience Private Limited’s Mycorrhizal Spores?
With climate change, water scarcity, and soil degradation on the rise, the need for biological resilience in agriculture has never been greater.
Mycorrhizal fungi that Agrilogy Bioscience Private Limited offer a natural, scalable solution:
- Reduce dependency on chemical fertilizers
- Improve soil carbon and organic matter content
- Enhance water-use efficiency
- Strengthen plants against stress and disease
These aren’t futuristic concepts—they're happening now, in fields around the world. And while the science continues to evolve, the practical applications are already transforming agricultural landscapes.
At Agrilogy Bioscience Private Limited, we don’t just provide any mycorrhizal spores—we offer premium, high-quality fungi that are optimized for different environmental conditions. Here’s why Agrilogy Bioscience Private Limited’s spores stand out:
- High-Quality Spores: Our spores are rigorously tested to ensure they are effective and reliable, helping you get the best results for your crops.
- Diverse Selection: From nutrient-rich soils to drought-prone regions, Agrilogy Bioscience Private Limited offers a diverse range of mycorrhizal species and blends to suit your unique agricultural needs.
- Compatibility: Our spores are compatible with a wide variety of crops, ensuring that you can use them in both conventional and organic farming systems.
- Sustainable Agriculture: By promoting healthier plants and better soil conditions, Agrilogy Bioscience Private Limited’s spores contribute to sustainable farming practices that reduce the need for chemical fertilizers and pesticides.
It’s Time to Look Below the Surface
As we transition toward more sustainable and regenerative models of agriculture, mycorrhizal fungi are emerging as a foundational tool in the toolkit.
They don’t just make plants grow—they help restore balance to ecosystems, increase crop resilience, and make farming more adaptive in the face of uncertainty.
So next time you look at a thriving plant, remember: its greatest allies may be the ones you can’t see.
Let’s stop treating soil like dirt.
Let’s treat it like the living, breathing ecosystem it truly is.
Get Started Today!
Ready to take the next step in soil health and crop productivity. Browse Our Selection and order your mycorrhizal spores today. Join the many farmers already reaping the benefits of healthier, more resilient crops with Agrilogy Bioscience Private Limited.
Rooted in Resilience: The Power of Mycorrhizal Technology
Farming on the Edge: Soil Degradation, Climate Stress, and What Comes Next?
Did you know that over 16% of India’s land is classified as barren?
According to a 2023 survey by the Department of Agriculture & Farmers Welfare, these lands—spanning deserts, degraded mountains, and drought-prone zones—are no longer suitable for cultivation. And the reason is range of stressors, both biotic and abiotic which include water scarcity, overuse of chemical fertilizers, poor agricultural practices such as excessive tillage, and soil contamination by heavy metals. Together, they lead to a decline in essential soil nutrients, loss of microbial biodiversity, increased soil erosion, and reduced agricultural productivity.
But what if we told you that a microscopically, hidden beneath our feet, holds the power to restore this land and reshape the future of farming? Welcome to the fascinating world of rootrrhiza—nature’s underground network that’s transforming how we grow food.
Understanding Mycorrhiza: Nature’s Underground Network
Mycorrhiza refers to a symbiotic association between plant roots and certain beneficial fungi. Derived from the Greek words for "fungus" and "root," mycorrhiza represents one of nature’s oldest and most efficient mutualisms. In this partnership, the fungus penetrates the plant's root system and extends into the surrounding soil through fine thread-like structures called hyphae. These hyphae increase the plant's access to water and nutrients, especially phosphorus, which are otherwise difficult to absorb. In exchange, the plant provides the fungus with sugars and lipids produced through photosynthesis. This mutually beneficial exchange not only enhances plant health but also promotes long-term soil sustainability.
Two Paths to Partnership: Endomycorrhizae vs. Ectomycorrhizae
There are two major categories of mycorrhizal fungi: endomycorrhizae (also known as arbuscular mycorrhizal fungi or AMF) and ectomycorrhizae.
Endomycorrhizae form internal associations with plant root cells and are known to colonize around 85% of terrestrial plant species, including most food crops. These fungi form specialized structures such as arbuscules (for nutrient exchange) and vesicles (for storage and reproduction).
Ectomycorrhizae, on the other hand, form external networks around root surfaces and are more commonly associated with woody plants and trees, including conifers and hardwoods. While most plants benefit from these associations, members of the Brassica family (such as cabbage, cauliflower, and mustard) are typically non-mycorrhizal.
Why AMF Matter: 7 Powerful Benefits for Modern Agriculture
Arbuscular mycorrhizal fungi (AMF) are not just biological allies—they are integral to enhancing crop resilience, soil health, and long-term agricultural productivity. The symbiosis between AMF and plant roots triggers a cascade of beneficial physiological and ecological responses that address both nutrient dynamics and environmental stresses. Below are the key agronomic advantages of incorporating AMF into modern farming systems:
- Enhanced Nutrient Absorption and Assimilation: AMF significantly increase a plant’s ability to absorb essential macro- and micronutrients, particularly phosphorus, from the soil. Their extensive hyphal networks function as an extension of the root system, accessing nutrients from soil pores that roots alone cannot reach. This leads to improved nutrient uptake efficiency and better assimilation of minerals, directly supporting plant growth and development.
- Resistance to Drought and Salinity Stress: Through improved water absorption and efficient resource allocation, AMF-inoculated plants exhibit enhanced tolerance to abiotic stresses like drought and soil salinity. These fungi help maintain plant hydration and osmotic balance during periods of water scarcity, enabling better survival and yield under challenging climatic conditions.
- Suppression of Pests and Pathogens via Secondary Metabolites: Mycorrhizal association stimulates the production of secondary metabolites in plants—compounds that play critical roles in defense against pests and pathogens. These biochemical changes reduce dependency on chemical pesticides by activating the plant's natural defense systems.
- Improved Soil Structure and Reduced Erosion: The hyphal network of AMF aids in soil particle aggregation, improving soil structure and porosity. This enhances aeration, water infiltration, and root penetration, while simultaneously reducing surface runoff and soil erosion—critical factors for maintaining long-term soil fertility.
- Reduced Nutrient Leaching and Increased Use Efficiency: AMF reduce the leaching of essential nutrients—especially phosphorus—from the soil. By capturing and recycling nutrients within the root-fungal system, these fungi improve nutrient-use efficiency and help retain valuable elements within the agricultural ecosystem.
- Promotion of Microbial Diversity and Soil Ecosystem Health: AMF foster symbiotic relationships with a range of other beneficial soil microbes, including nitrogen-fixing bacteria. This cooperative environment leads to a more balanced and productive soil microbiome, essential for sustaining high-quality organic farming systems.
- Soil Decontamination and Pollution Mitigation: Mycorrhizal fungi play a pivotal role in the detoxification of soils contaminated by heavy metals and synthetic chemicals. By binding pollutants and enhancing nutrient cycling, AMF contribute to cleaner soil environments, facilitating the decomposition of organic matter and promoting overall soil health.
Together, these agronomic benefits underscore the strategic importance of AMF in building resilient, high-performing agricultural systems. By integrating Rootrrhiza inoculants into crop management practices, farmers can move toward sustainable agriculture that is both productive and ecologically responsible.
As agriculture navigates the twin pressures of soil degradation and climate variability, biological alternatives have emerged as both practical and essential. Rootrrhiza offer a resilient, eco-friendly solution that integrates seamlessly into organic and regenerative farming practices.
By improving nutrient efficiency, enhancing plant stress tolerance, and rebuilding soil microbial health, Rootrrhiza inoculants play a central role in restoring ecological balance while increasing productivity.