
- Home
- The Complexity Behind MTT Staining: Navigating Variability in Spore Viability Testing

Aditi Bijalwan
Co-founder at Agrilogy Bioscience Private Limited
When it comes to assess the viability of fungal spores (especially VAM Fungi), the MTT assay seems like a simple solution—just add the dye and observe the colour change. But what happens when the colour reactions are anything but straightforward? Dive into the controversies and complexities behind MTT staining and discover why accurate interpretation of spore viability is trickier than it seems.
Assessing the viability of fungal spores, particularly those from arbuscular mycorrhizal fungi (AMF or VAM), is essential for understanding their role in plant symbiosis.
One widely used method for evaluating spore viability is the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide) assay. This assay measures the metabolic activity of spores, offering a visually accessible indicator of their health. However, despite its popularity, MTT staining can lead to inconsistent and sometimes controversial interpretations. Let’s break down the principles behind MTT, its colour interpretations, and the factors that cause variability in results.
What is MTT Dye?
MTT is a yellow tetrazolium dye that is reduced by cellular dehydrogenases, enzymes that play a critical role in cellular respiration. When the dye is reduced in respiring cells, it forms a purple formazan product. The intensity of the purple colour directly correlates with the metabolic activity of the cells or spores, making MTT a useful tool for assessing cell viability. For VAM spores, this process indicates whether the spores are metabolically active and potentially viable for symbiosis with plant roots.
How Does MTT Work?
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) staining is a widely used assay to assess cell viability based on metabolic activity. When introduced to living AMF or VAM cells, MTT is reduced by mitochondrial dehydrogenase enzymes, which disrupt the tetrazolium ring structure and form a purple-coloured formazan product. This reaction occurs in metabolically active cells where enzymes, aided by cofactors like NADH or NADPH, facilitate the reduction process.
The resulting formazan accumulates inside the cytoplasm, and the intensity of the colour produced correlates directly with the cellular metabolic activity of the spore. The ease with which MTT passes through the lipid membranes of viable cells ensures its effective uptake, making it an excellent indicator of cell health and viability. The amount of formazan formed is a quantitative measure, offering a reliable insight into the viability of cells or spores, such as VAM spores, by highlighting their metabolic capacity.
MTT Assay Protocol for Spore Viability Assessment:
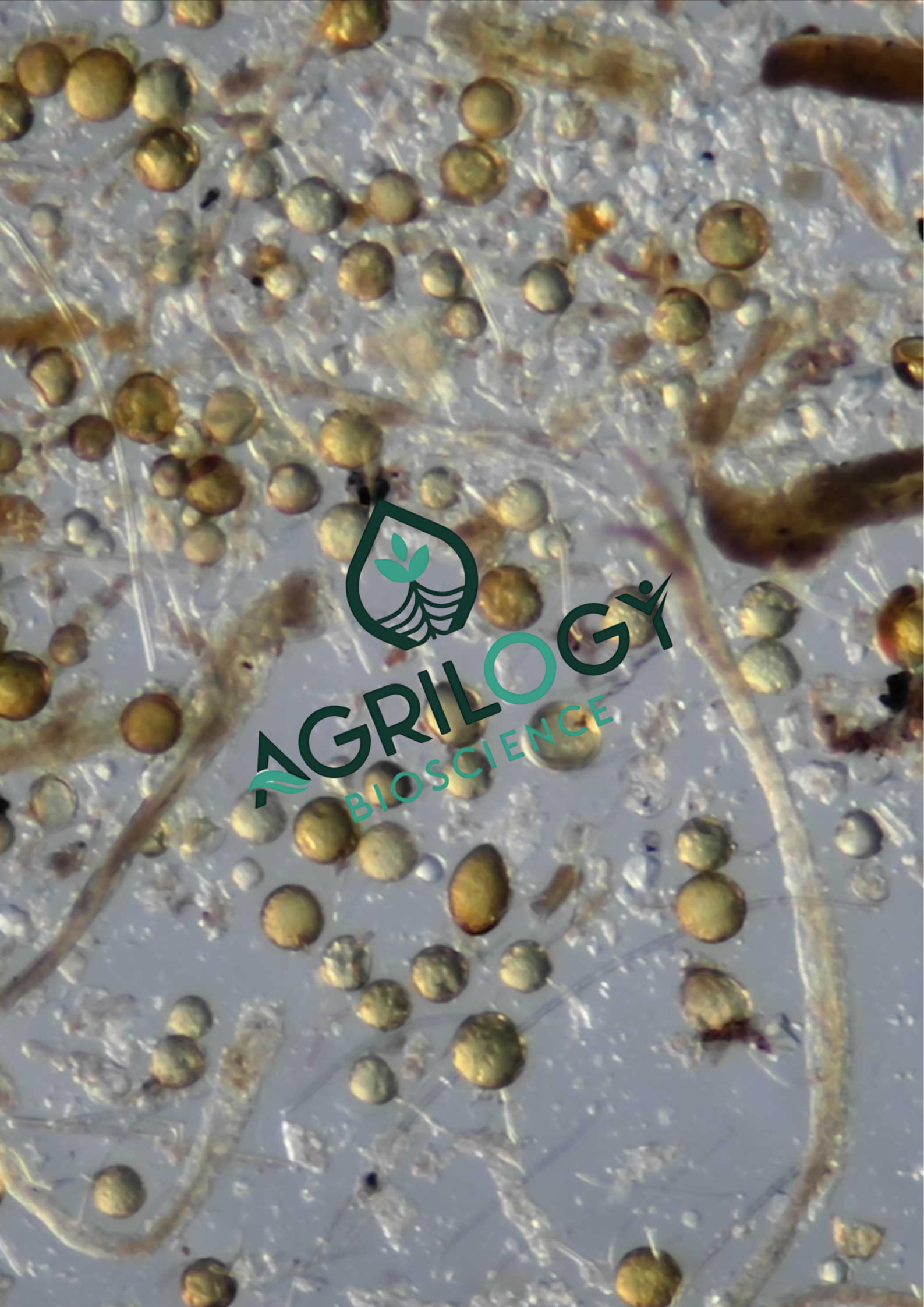
The MTT Assay Protocol involves weighing 1 gram of the sample (e.g., fungal spores) and washing it under tap water multiple times. The collected spores are diluted in freshly prepared 0.25% MTT dye. The mixture is then inverted to ensure thorough mixing and incubated at 27°C in the dark for 24, 48, and 72 hours. After incubation, the sample is washed 3-4 times with tap water and then suspended in 10 ml of RO water. A 0.5 ml aliquot is placed on a petri dish and examined under a stereo zoom microscope for spore viability.
Colour Interpretation and Its Significance:
The colour changes in MTT staining offer critical insights into spore viability. However, these changes are not always straightforward:
- Red/Pink/Purple Spores: Viable spores typically stain red, pink and purple with the intensity of the colour corresponding to the level of metabolic activity. A purple colour indicates active dehydrogenase enzymes, while a pink hue suggests lower metabolic activity.
- Black Spores: Black spores are often assumed to be non-viable, as they suggest excessive formazan accumulation in the cytoplasm upon reduction by cellular reducing agents. However, black coloration may also result from overstaining, leading to ambiguities in viability interpretation.
- Unstained Spores: Chemically killed or non-metabolically active spores will not reduce MTT, and thus remain yellow, indicating non-viability.
Despite these general rules, variability in results often complicates the interpretation of spore viability.
Controversies and Sources of Variability in MTT Staining:
Although MTT staining is widely used, its application comes with notable challenges. Here are some key factors that contribute to its variability:
1. Overstaining and Misleading Black Spores:
✓Black coloration in MTT staining is commonly linked to non-viability, but this can be misleading. Excessive MTT accumulation or prolonged incubation can result in black spores, even if they are still viable. Therefore, black coloration should not be automatically interpreted as a sign of non-viability.
2. Impact of Spore Age and Wall Thickness:
✓Older spores, particularly those that have been stored for extended periods, require higher concentrations of MTT or longer incubation times to reach a maximum colour change. Furthermore, the thickness of the spore wall can affect the penetration of MTT. Thicker walls may limit the dye’s access to the enzymatic sites, resulting in inconsistent staining and variability in colour reaction.
3. Dormant Spores and Metabolic Decline:
✓Spores stored for long periods often experience a decline in metabolic activity. These dormant spores may still reduce MTT, producing faint red or pink hues despite being non-viable in terms of germination or colonization. This presents a challenge in distinguishing between dormant and truly viable spores, as they may still display metabolic activity despite not being capable of establishing symbiosis.
4. Chemical Reducing Agents in Non-Viable Spores:
✓Non-viable spores may still reduce MTT due to the presence of reducing agents like cysteine, sulphur-containing compounds, or reducing sugars. These chemicals can catalyse the reduction of MTT even in the absence of active dehydrogenase enzymes, leading to false positives and incorrect conclusions about spore viability.
5. Species-Specific Variability:
✓Different VAM species exhibit varying metabolic activity levels. As a result, their responses to MTT can differ significantly. Some species may require longer incubation or higher concentrations of MTT to achieve a visible colour change, making standardization across species challenging.
6. Difficulties in Interpreting Pink Spores:
✓Pink spores can be particularly problematic. A faint pink colour may suggest minimal metabolic activity, but it is difficult to determine whether the spores are dormant or in a state of partial viability. These spores may be capable of reducing MTT but lack the resources to germinate or colonize plant roots.
Conclusion: Navigating the Complexities of MTT Staining:
While MTT staining is a useful and widely employed technique for assessing the viability of fungal spores, it is far from perfect. A thorough understanding of its limitations is essential for accurate interpretation. Factors such as overstaining, spore wall thickness, spore age, and the presence of reducing agents must be carefully considered when analysing the results. In particular, the ambiguous interpretation of black or pink spores underscores the need for caution in drawing conclusions based solely on MTT staining.
To minimize variability and ensure reliable results, optimization of experimental conditions is essential. Researchers must account for species-specific characteristics, spore age, and other confounding factors in order to achieve accurate assessments of fungal spore viability. In summary, MTT staining is a valuable tool, but its complexity requires a refined approach for obtaining meaningful data.
Key Takeaways:
- MTT staining helps assess spore viability by measuring metabolic activity through colour changes.
- Red or pink colouration typically indicates viable, respiring spores, while black colouration can indicate overstaining or non-viability.
- Factors like spore age, wall thickness, and the presence of reducing agents can affect MTT staining results, leading to potential misinterpretations.
- Optimization of experimental conditions is crucial for accurate viability assessment across different VAM species.

Comments (0)
No comments yet. Be the first to comment!




Leave a Comment